Some of my readers know me for my tweets about #OrganicElectronics. In this field, fullerene and its derivative have been for a long time used in many applications for their conductive properties. Last December, I was intrigued by a paper about the use of fullerene for perovskite interlayers: Cross-Linkable Fullerene Derivatives for Solution-Processed n–i–p Perovskite Solar Cells. In this post, I want to show you how this research is inspiring!
I’ve been in contact with one of its authors, Guillaume Wantz, Professor at the University of Bordeaux (France), since then. I wanted to invite him to present here his work and his views about the use of fullerene derivatives in OPV and perovskite solar cells. We shared a very interesting conversation leading to this blog post.
What is fullerene and why is it fabulous?
Neat fullerene is a conjugated sphere of 60 carbons. It is an amazing electron acceptor material. That is why we use its derivatives, such as PCBM, in Organic Photovoltaics. In the bulk heterojunction, it is usually blended with an electron donating polymer. It helps the charge separation and ensures the exciton does not recombine. However, the mechanism is not clear. Most of past studies with fullerene in solar cells are empirical: scientists observed fullerene is a good material without bringing theoretical hypothesis. Maybe someone knows why it is that good, but no one revealed it! I predict the understanding of its physical properties in the bulk heterojunctions will become a research field of paramount importance.
For now, what we can be sure about fullerene is that it is a very versatile molecule. We can crosslink it, photo-oxydize it, or anything! That is exactly what Guillaume did.
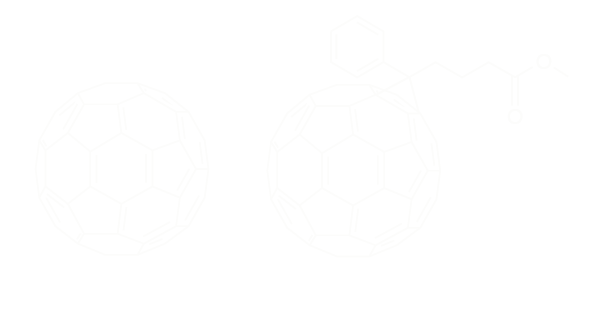
Proceeding by trials and errors
Research is really interesting, for that it is full of twists and turns. It is a tortuous path towards a finding and discoveries. About his paper, Guillaume tells it like anyone else:
Some years ago, within the ELORGA group in Bordeaux, we had developed C60 crosslinking strategies for organic photovoltaics. Our idea was to freeze the nanometric morphology of bulk heterojunctions thanks to crosslinking. To do so, we had worked for several years trying to bind C60 one on each other in situ − a kind of polymerization of C60. We finally came up with some azide derivatives to be blended with PCBM for a stable morphology.
Prof. Henry Snaith (Oxford) read our study and contacted us. At the time, he was looking for some crosslinkable C60 derivatives to use as interlayer in his perovskite solar cell. Crosslinked because he wanted to avoid the dissolution of the interlayer by the subsequently coated layers. The project was to coat a layer of fullerene and then make it insoluble before continuing the construction of the solar cells. In fact, there is a real problem with solubility. Neat C60 it is not really soluble, one cannot coat it sucessfully without making pellets. That is why derivatives, like PCBM, that are more soluble are better for this application. However, they can diffuse and their solubility may affect chemically the layers of the solar cell.
Firstly tried, the azide crosslinkers chemistry affected the C60 structure and lowered its electron accepting ability. Thus we proposed a novel sol-gel processed C60 coming from Dr. Olivier Dautel, an inspiring CNRS reasearcher from Montpellier. I did not know what to do with this material before! In 5 years, we kept this material in our “drower of unsuccessful materials” until having the problem with azide crosslinkers. I had the idea to make sol-gel processable layers that could be insoluble. Eureka! It works very well!
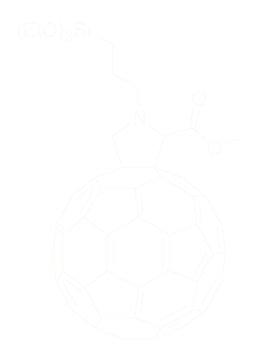
Nonetheless, this material is not really easy to process: the way we had to think the sol-gel catalysis was not obvious. The initialization of the reaction must be done with acid to start the hydrolysis, for the polycondensation to happen. Imagine a real sol-gel ink to be spin-coated, it is reactive and will evolve eventually quickly. The truth is you never know what you’re spin-coating! Then we had the idea to spin-coat the pristine molecules, to make a layer and then expose it to acid vapors for initializing the hydrolysis. We used a soft acid not to ruin the ITO electrode: we exposed the fullerene layer to trifluoroacetic acid vapors. It was successful, films were finally insoluble in every organic solvents.
The real problem is that during the sol-gel process, SiOx moities are created. These are neither conducting nor semiconducting, they are the essence of the known glassy insulator (SiO2). We carried out mobility measurements on organic field effects transistors and on vertical sandwiched structures using SCLC models. When comparing to PCBM, we lost 2 orders of magnitude in term of electrons mobility! That was astonishing. However, the result was not that bad on vertical sandwichs. That is the clue! We use just a thin interlayer of that material so that the carriers do not travel long distance through it.
Then they managed to put only a tiny part of fullerene that worked well for the application in n-i-p perovskite solar cells! Is that not exciting?
Beyond Fullerene
Following this article and the work that was done, one might want to go further. But that needs a deeper understanding of the phenomena that make fullerene that versatile. One could think about carbon nanotubes: they are a good electron acceptor, fitting the requirement we outlined with good conducting properties. However, carbon nanotubes are harshly processable. Many tried to make films with it. We do not believe in it for the OPV applications as fullerene substitutes.
Nevertheless, as we did with the sol-gel C60, one can find new molecules, non-fullerene acceptors (NFA). As with many other groups in the world, Guillaume is on this new promising topic!
To conclude, there is a real problem with fullerene: it does photo-oxide on its cage in presence of oxygen. Then when one processes it at an industrial scale, it is necessary to expose it to the air. Thus a tiny part of oxygen can fix the cell and, one day will kill it. We don’t know when. That is the topic of Guillaume’s forthcoming article, which we are eagerly awaiting.
Contact information:
Prof. Guillaume Wantz, Bordeaux Institute of Technology (Bordeaux INP / ENSCBP) guillaume.wantz@ims-bordeaux.fr
The molecules representations are courtesy of Prof. Guillaume Wantz.
 0000-0002-6484-2157
0000-0002-6484-2157